Vaccines arriving, Health Ministry emphasises their safety and efficacy
Malaysia’s first batch of Covid-19 vaccines, from Pfizer-BioNTech, is set to arrive in the country this Sunday morning (Feb 21).
Putrajaya previously said it intends to vaccinate at least 70 percent of the population in order to achieve herd immunity.
However, the government may face challenges in reaching this target as recent surveys by the Health Ministry showed that only about 67 percent of respondents said they would agree to the vaccination.
Meanwhile, another 17 percent said they are on the fence while 16 percent said they would reject it.
At the time, Health Ministry director-general Dr Noor Hisham Abdullah (above) acknowledged the public’s concerns surrounding the safety, efficacy and side effects of the Covid-19 vaccines.
As such, in a three-hour-long press briefing held via video conference organised by the Health Ministry yesterday, Noor Hisham and the ministry’s experts attempted to address these concerns.
He said the ministry will focus on convincing the 17 percent who are on the fence when it comes to the vaccine.
“We have to convince them about the safety and effectiveness of the vaccine,” Noor Hisham said.
Health Ministry Clinical Research Institute director Dr Kalaiarasu Peariasamy, who gave a presentation about research regarding the safety and efficacy of Covid-19 vaccines, said many people assume the vaccine developments were rushed.
“(But) we have to recognise why the speed was so fast in vaccine delivery (for Covid-19),” he said.
This was because the research for Covid-19 vaccines received huge amounts of funding from governments around the world as well as non-governmental organisations and other sources of donations, Kalaiarasu said.

Also, he explained, cases are required in order for vaccines to be tested and usually, researchers may have to wait a long time for cases.
In the case of Covid-19, he said, there were a large number of cases to be tested, which increased the researchers’ speed.
Kalaiarasu also stressed that all clinical trials were conducted following international standards and that there were none where humans were subjected to trials with a high risk of death.
“This is enforced very strictly in vaccine trials,” he said.
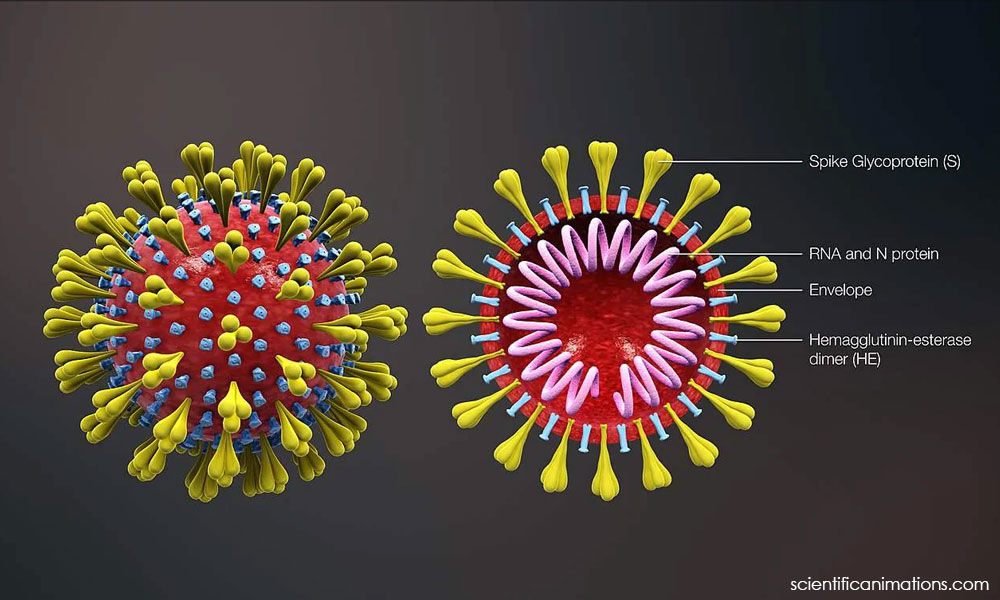
There have also been concerns about the new types of platforms being used in the Covid-19 vaccines, such as the mRNA platform utilised by the Pfizer-BioNTech and Moderna vaccines and the viral vector used by the AstraZeneca and Sputnik V vaccines.
Kalaiarasu explained that the mRNA vaccines are merely designed to produce a certain type of protein in the body.
“Once (the vaccine) is received, the molecule that is injected is only designed to produce protein, not to produce coronavirus or to stick to any part of the body.
“This protein resembles a (coronavirus) spike protein and this is how to develop specific antibodies and what we call T-cells,” he said.
Meanwhile, the viral vector, such as the one used by AstraZeneca, has a harmless cold virus act as a delivery system (vector) for the genetic sequence of the spike protein, he explained.
Kalaiarasu also addressed worries over possible side effects after receiving the vaccination.
“The vaccines had side effects even during the trial, there were enough reports of local pain and swelling, fever, fatigue, and headache.
“Many of these were temporary, lasting over one to two days and almost all of them resolved within four to five days.
“There were no situations where these side effects became a reason to abandon the trials. (The side effects) were not considered severe enough for regulatory bodies to take action,” he said.
He added that there have been at least four Covid-19 vaccines which were abandoned before finishing trials.
People should remember that most vaccines, even for other illnesses, have side effects as your body is reacting to the vaccine, he said.
However, Kalaiarasu did acknowledge reports of severe allergic reactions (anaphylaxis) to mRNA vaccines since the vaccines started being administered in other countries last December.
He noted that it was a very small number of cases and most who experienced this had a prior history of anaphylaxis.
“Someone who has severe allergies will be aware of it and of their prior history. They need to announce this to the medical officers who will run the vaccination.
“They are not recommended to take the (mRNA) vaccine based on their anaphylaxis history.
“It is very, very rare that it affects those without a history of anaphylaxis,” he said, adding that it usually occurs within half an hour of the vaccination.
The Health Ministry is also prepared to deal with emergency treatment if such an incident were to occur, he said.
For those worried about regulatory requirements for the vaccines, Kalaiarasu said the National Pharmaceutical Regulatory Agency (NPRA) requires it to be at least 50 percent effective.
The NPRA also requires safety data from more than 3,000 study participants for at least two to three months.
What this means, he said, is that the NPRA requires the trials to continue monitoring their participants for at least two to three months after their last vaccine dose for any other side-effects.
Kalaiarasu urged members of the public to get vaccinated as this would help protect the vulnerable and senior citizens in the community.
The government previously released details of its three-phase vaccination plan, with the first phase focusing on medical frontliners, defence frontliners and other “essential service” frontliners from February to April 2021.
The second phase is set to take place from April to August 2021, with a focus on vaccinating the remaining healthcare and defence personnel as well as senior citizens above 60, people with disabilities, and other high-risk groups with co-morbidities.
Finally, the last phase from May 2021 to February 2022 will provide vaccinations to both citizens and non-citizens above 18 years old, with priority given to those residing in red zones, followed by yellow zones and lastly, to those in green zones. - Mkini
✍ Credit given to the original owner of this post : ☕ Malaysians Must Know the TRUTH
🌐 Hit This Link To Find Out More On Their Articles...🏄🏻♀️ Enjoy Surfing!



















Post a Comment